Oct 16,2025
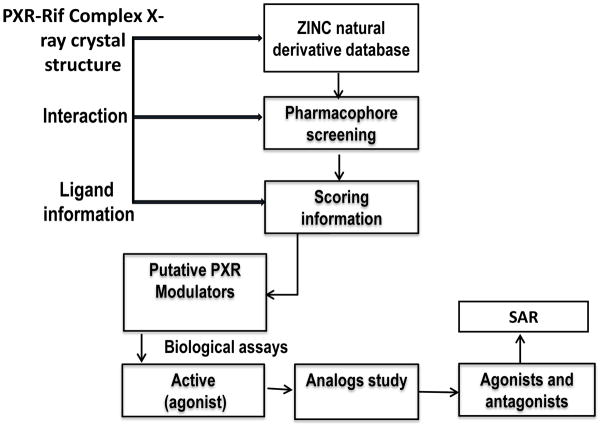
靶向PXR:天然产物衍生物调控药物代谢新策略!本研究中蛋白表达与纯化通过美迪西进行
Nuclear receptors are ligand-activated transcription factors involved in regulating many physiologic and pathologic processes. Ligand binding to nuclear receptors leads to dissociation of co-repressors, recruitment of co-activators, and subsequent activation of gene expression. Because of their associations with many human diseases, nuclear receptors are therapeutic targets for pharmaceutical development.
The pregnane X receptor (PXR, NR1I2) belongs to the nuclear receptor family. The activity of PXR can be controlled by the binding of small molecule agonists or antagonists. Because of its unique ligand binding pocket, PXR binds promiscuously to structurally diverse chemicals.
PXR regulates the expression of proteins such as drug metabolizing enzyme cytochrome P450 3A4 (CYP3A4), efflux transporter P-glycoprotein, and other multidrug resistance proteins, which are involved in metabolism and elimination of potentially harmful chemicals. PXR has been detected in various tissues including kidney, colon, brain capillaries, small intestine, and predominantly in liver, and it can be activated by various ligands that bind to its ligand binding domain (LBD). PXR forms a heterodimeric complex with RXR to activate gene transcription.
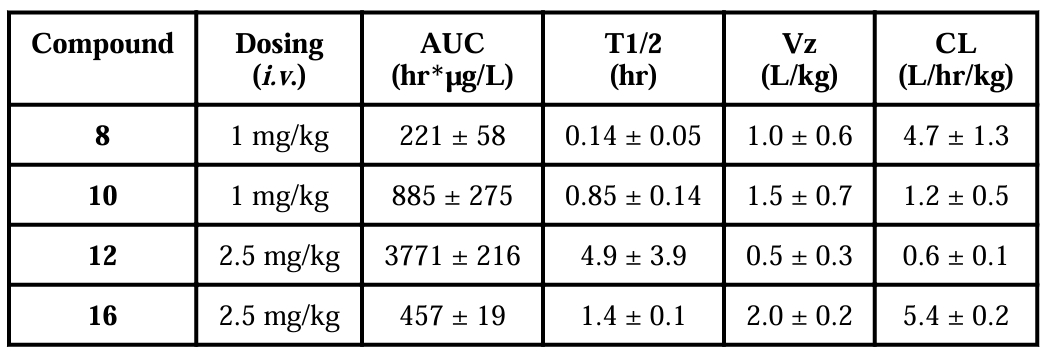
The recombinant pETDuet1-hPXR-LBD/mSRC-1 plasmid (Medicilon, Shanghai, China) was transformed into Escherichia coli BL21 DE3 cells for protein expression.
查看更多