Oct 15,2025
卓越成药性:高效AR-PROTAC降解剂ARD-2051在多个物种中展现优质PK属性与口服生物利用度!本研究中PK实验通过美迪西进行
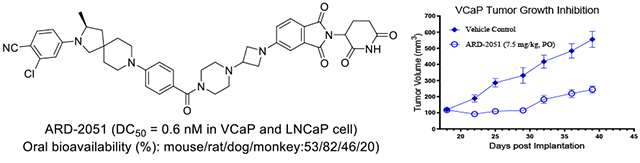
Prostate cancer (PCa) is a major global health concern. The androgen receptor (AR), a key member of the nuclear hormone receptor superfamily, is vital for prostate gland development and the maintenance of male secondary sexual characteristics. ARD-2051 is a potent and orally bioavailable AR PROTAC degrader.
In vitro microsomal metabolic stability studies of AR degraders were performed in Medicilon.
The in vitro plasma stability of a test compound was studied in human, mouse, rat, dog and monkey plasmas in Medicilon.
Plasma protein binding of a test compound was assessed by equilibrium dialysis method with dialysis membrane strips in Medicilon.
The CYP inhibition of a test compound was studied in human liver microsomes in Medicilon.
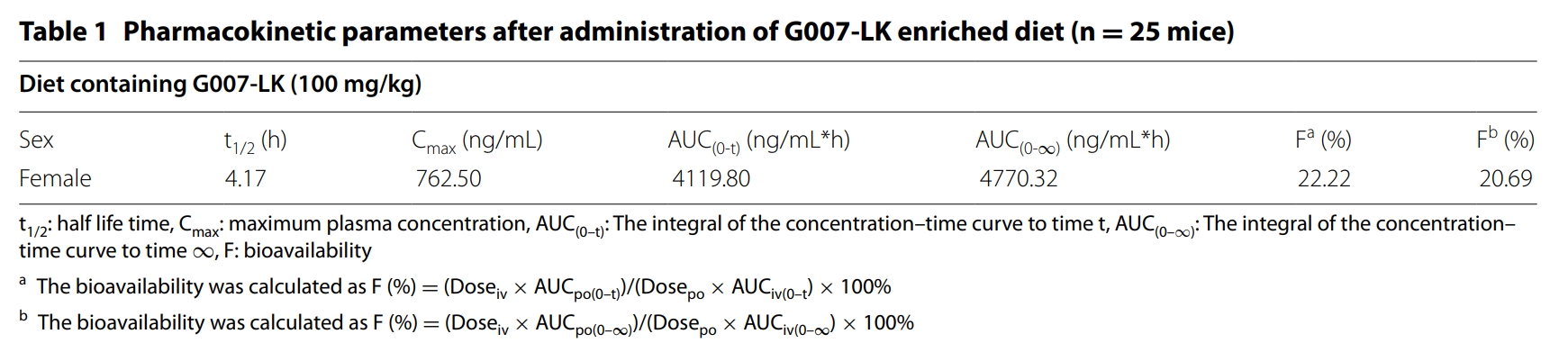
PK studies in mice, rats and dogs were performed in Medicilon.
In mouse, ARD-2051 has an excellent overall PK profile, with a low clearance (Cl = 3.7 ml/min/kg), a moderate volume of distribution (Vss = 1.3 L/kg), a half-life of approximately 5 h with both IV and oral routes of administration, and an excellent oral exposure with a good oral bioavailability (F) of 53%.
In rat, ARD-2051 has a low-moderate clearance in rats (Cl = 10.2 ml/min/kg), a moderate volume of distribution (Vss = 1.3 L/kg), a half-life of 2-2.3 h with intravenous (IV) and oral routes of administration, and achieves an excellent oral bioavailability (F) of 82%.
In dog, ARD-2051 has a low-moderate clearance (Cl = 4.6 ml/min/kg), a good volume of distribution (Vss = 2.8 L/kg), a long half-life of 8.9 h with IV route of administration and 21.1 h with oral route of administration, an excellent oral exposure (Cmax = 294 ng/ml and AUC=1822 h*mg/ml at 1 mg/kg), and an oral bioavailability (F) of 46%.
These data indicate that ARD-2051 has favorable pharmacokinetic properties and excellent oral bioavailability in mice, rats, and dogs.
查看更多