



业务咨询
中国:
Email: marketing@medicilon.com.cn
业务咨询专线:400-780-8018
(仅限服务咨询,其他事宜请拨打川沙总部电话)
川沙总部电话: +86 (21) 5859-1500
海外:
+1(781)535-1428(U.S.)
0044 7790 816 954 (Europe)
Email:marketing@medicilon.com




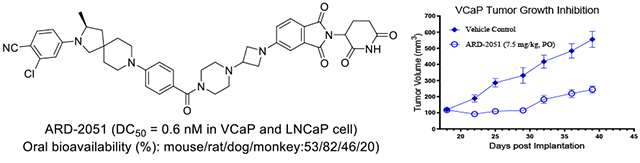
Prostate cancer (PCa) is a major global health concern. The androgen receptor (AR), a key member of the nuclear hormone receptor superfamily, is vital for prostate gland development and the maintenance of male secondary sexual characteristics. ARD-2051 is a potent and orally bioavailable AR PROTAC degrader.
 Microsomal Metabolic Stability Assay
Microsomal Metabolic Stability Assay
In vitro microsomal metabolic stability studies of AR degraders were performed in Medicilon.
Plasma Stability Assay
The in vitro plasma stability of a test compound was studied in human, mouse, rat, dog and monkey plasmas in Medicilon.
Plasma Protein Binding Assay
Plasma protein binding of a test compound was assessed by equilibrium dialysis method with dialysis membrane strips in Medicilon.
CYP Inhibition Assay
The CYP inhibition of a test compound was studied in human liver microsomes in Medicilon.
PK Studies in Mice, Rats, and Dogs
PK studies in mice, rats and dogs were performed in Medicilon.
 In mouse, ARD-2051 has an excellent overall PK profile, with a low clearance (Cl=3.7 ml/min/kg), a moderate volume of distribution (Vss=1.3 L/kg), a half-life of approximately 5 h with both IV and oral routes of administration, and an excellent oral exposure with a good oral bioavailability (F) of 53%.
In mouse, ARD-2051 has an excellent overall PK profile, with a low clearance (Cl=3.7 ml/min/kg), a moderate volume of distribution (Vss=1.3 L/kg), a half-life of approximately 5 h with both IV and oral routes of administration, and an excellent oral exposure with a good oral bioavailability (F) of 53%.
In rat, ARD-2051 has a low-moderate clearance in rats (Cl=10.2 ml/min/kg), a moderate volume of distribution (Vss=1.3 L/kg), a half-life of 2-2.3 h with intravenous (IV) and oral routes of administration, and achieves an excellent oral bioavailability (F) of 82%.
In dog, ARD-2051 has a low-moderate clearance (Cl=4.6 ml/min/kg), a good volume of distribution (Vss=2.8 L/kg), a long half-life of 8.9 h with IV route of administration and 21.1 h with oral route of administration, an excellent oral exposure (Cmax=294 ng/ml and AUC=1822 h*mg/ml at 1 mg/kg), and an oral bioavailability (F) of 46%.
These data indicate that ARD-2051 has favorable pharmacokinetic properties and excellent oral bioavailability in mice, rats, and dogs.
Reference:
 相关新闻
相关新闻